Molecular Water Oxidation Catalysts
Our work on molecular OER catalysts complements the quest for universal optimization strategies of transition metal centers. We have a long-standing interest in bio-inspired molecular catalysts with cuboidal metal cores which mimic both Nature’s {CaMn4O5} core of Photosystem II and crucial motifs of solid catalysts, such as spinels. They conveniently bridge the solid with the molecular catalysis realm.
We started from polyoxometalate (POMs) OER catalysts and proceeded with further investigations into their complementary HER properties (ChemPlusChem 2015, 80, 1389), structural dimensionalities (Inorg. Chem. 2017, 56, 327), and transitions to salt-like catalytic composites (Front. Chem. 2018, 6, 302).
Our earlier works on small bio-mimetic oxo clusters were focused on the translation of key PSII features into readily accessible {Co(II)4O4} cubanes. For example, we introduced both flexible ligand environments and redox-inert metal centers into {Co(II)3Ln} cubane OERs (J. Am. Chem. Soc. 2015, 137, 11076) to analyze the favorable effect of this structural tuning.
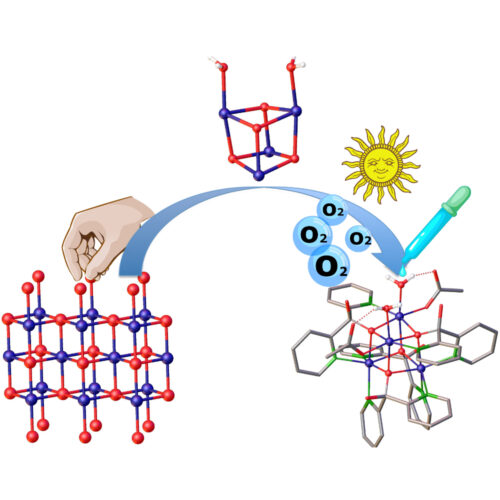
J. Am. Chem. Soc. 2017, 139, 14198
Intrigued by the structural similarities between Co/Ni-cubanes and oxide-based OERs, we constructed specific {(Co/Ni)(II)4O4} OERs with the characteristic “edge-site” reactive surface motif of oxides as their molecular cut-outs (J. Am. Chem. Soc. 2017, 139, 14198). This enabled us to explore central challenges, namely (1) Connections between molecular nuclearity and activity; (2) Different metal-metal synergies in molecules vs. solids, and (3) Predictive design of small oxo cluster types.
The fascinating influence of counteranions on the solution-based assembly of different {(Co/Ni)(II)4O4} cubane types came to light in an extensive screening study (J. Am. Chem. Soc. 2019, 141, 8846). Along with computational approaches (cooperation with Prof. Sandra Luber, UZH), we proposed the first formation pathways for different cubane types in solution – covering “terra incognita” in predictive coordination chemistry design.